Dr. Zhifeng Huang’s collaborative work on photoinduced asymmetric synthesis was published in Nature Chemistry
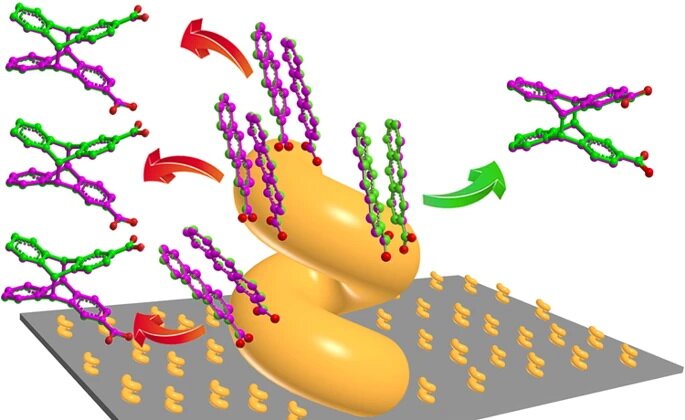
In general, chiral molecules are stereoselective synthesized through the mediation with another chiral molecules in the industry or laboratory, a process so-called asymmetric synthesis. To obtain molecular enantiopreference (or enantioselectivity) in the absence of any chiral molecule is greatly challenging and of fundamental interest toward better understanding of the homochirality, meaning that bio-molecules naturally occur in only one of two stereoisomeric configurations. Recently, Chinese researchers from three groups (Prof. Cheng YANG’s group at Sichuan University, Prof. Yanggang WANG’s group at Southern University of Science and Technology, and Prof. Zhifeng HUANG’s group at Hong Kong Baptist University) have collaboratively demonstrated that macroscopic shear forces, applied by glancing angle deposition (GLAD), enable the manipulation of molecular chirality through a “split-step chirality transfer” strategy. Metal nanohelices with right and left handednesswere deposited by GLAD onto a substrate rotated clockwise and counterclockwise, respectively. Then, a prochiral molecule, 2-anthracenecarboxylic acid (AC), was adsorbed on the metal nanohelices and photolyzed, to produce anti-head-to-head chiral cyclodimers withan enantiopreferential chirality that was reliably determined by the helical chirality of the metal nanohelices. The observed enantioselectivity mainly arose from the enantioselective spatial matching of the facially stacking AC dimers at the helical surfaces, revealed by the density functional theory calculations, and possibly from the chiroplasmonic effects.
This work demonstrates the unprecedented manipulation of molecular chirality indirectly through macroscopic control, and provides a brand new example of absolute asymmetric synthesis.
https://www.nature.com/articles/s41557-020-0453-0